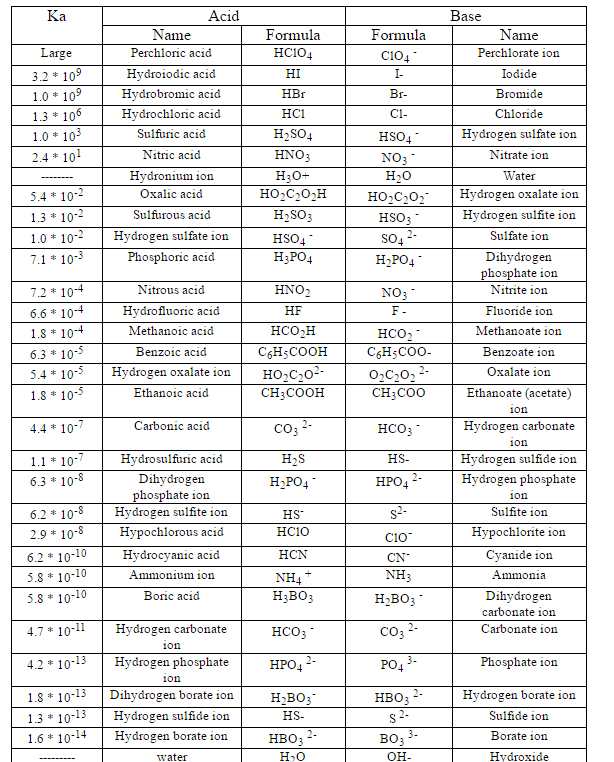
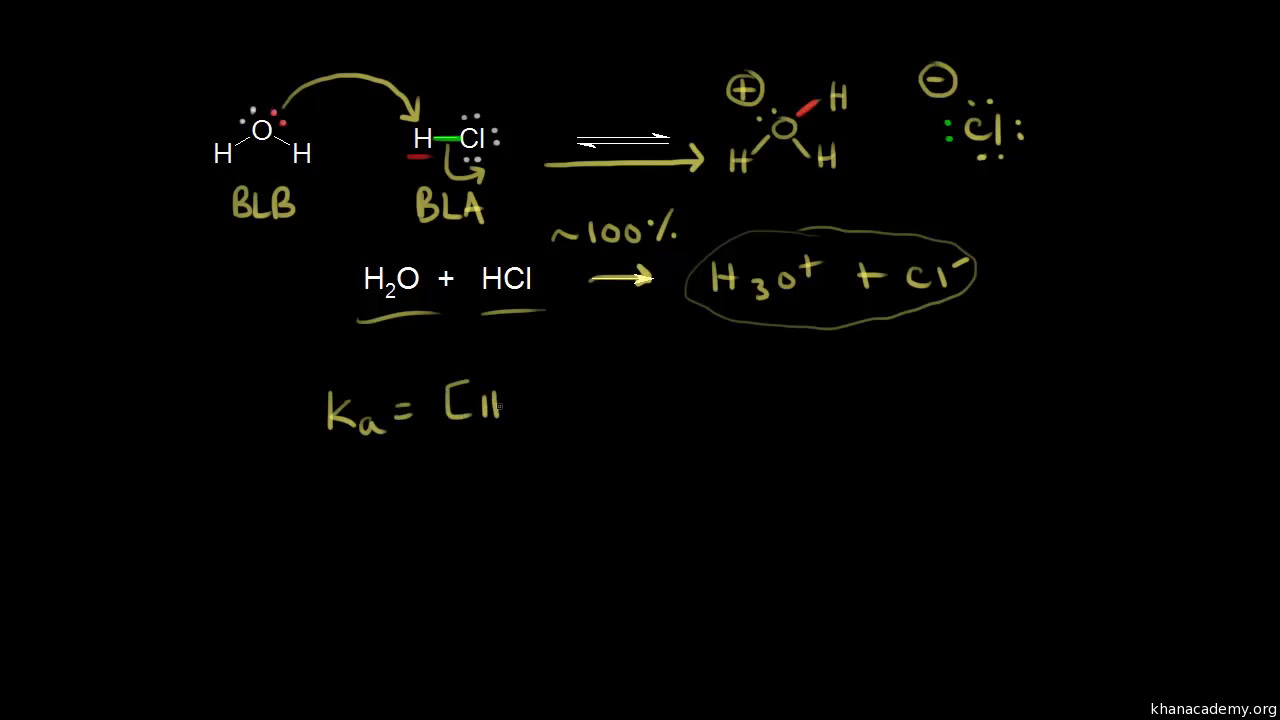
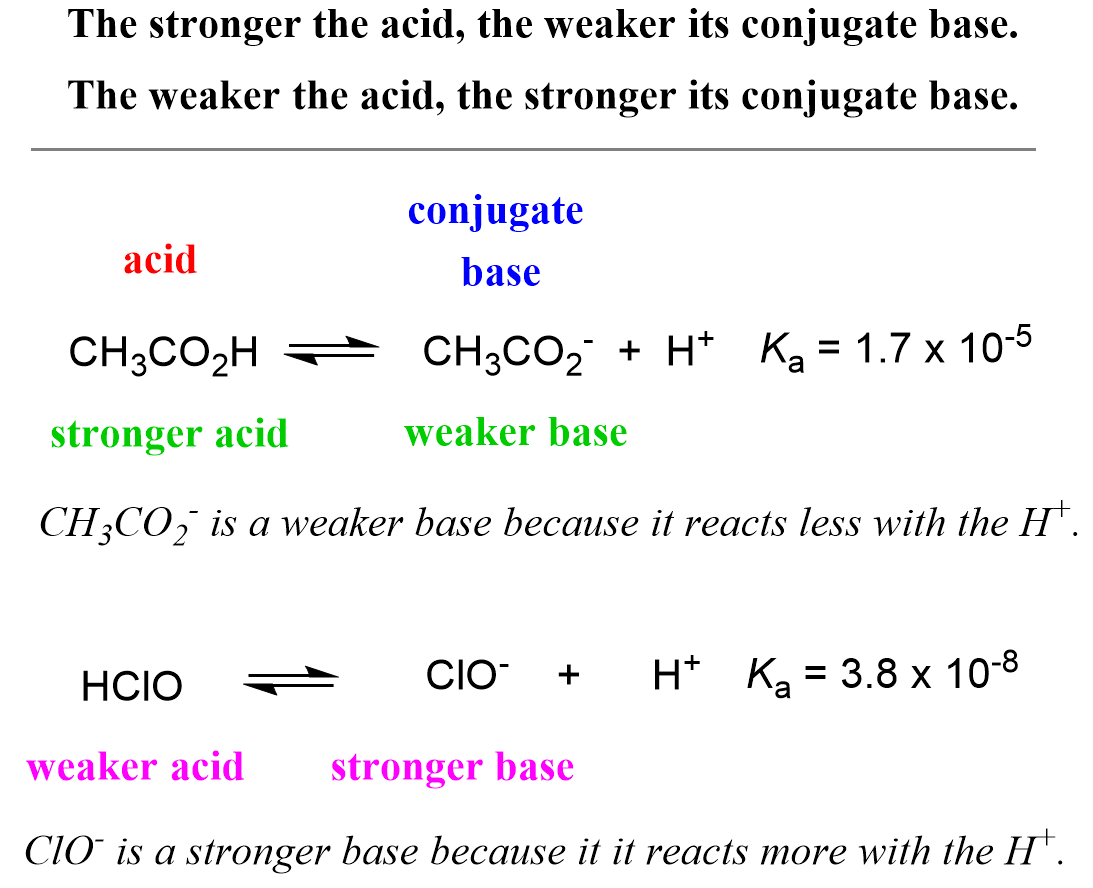
Lecture 1: Introduction and review –Quiz 1 –Website: –Review of acid/base chemistry –Universal features of. - ppt download
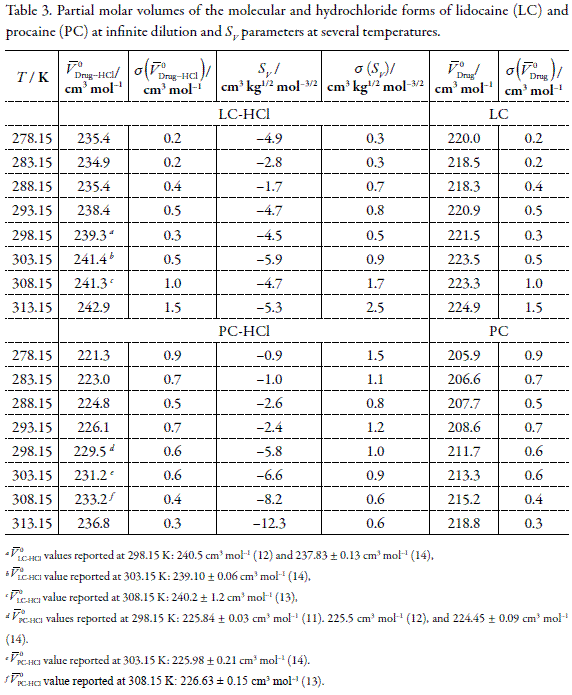
Apparent molar volumes of the anesthetic drugs procaine-HCl and lidocaine- HCl in water at temperatures from 278.15 to 313.15 K

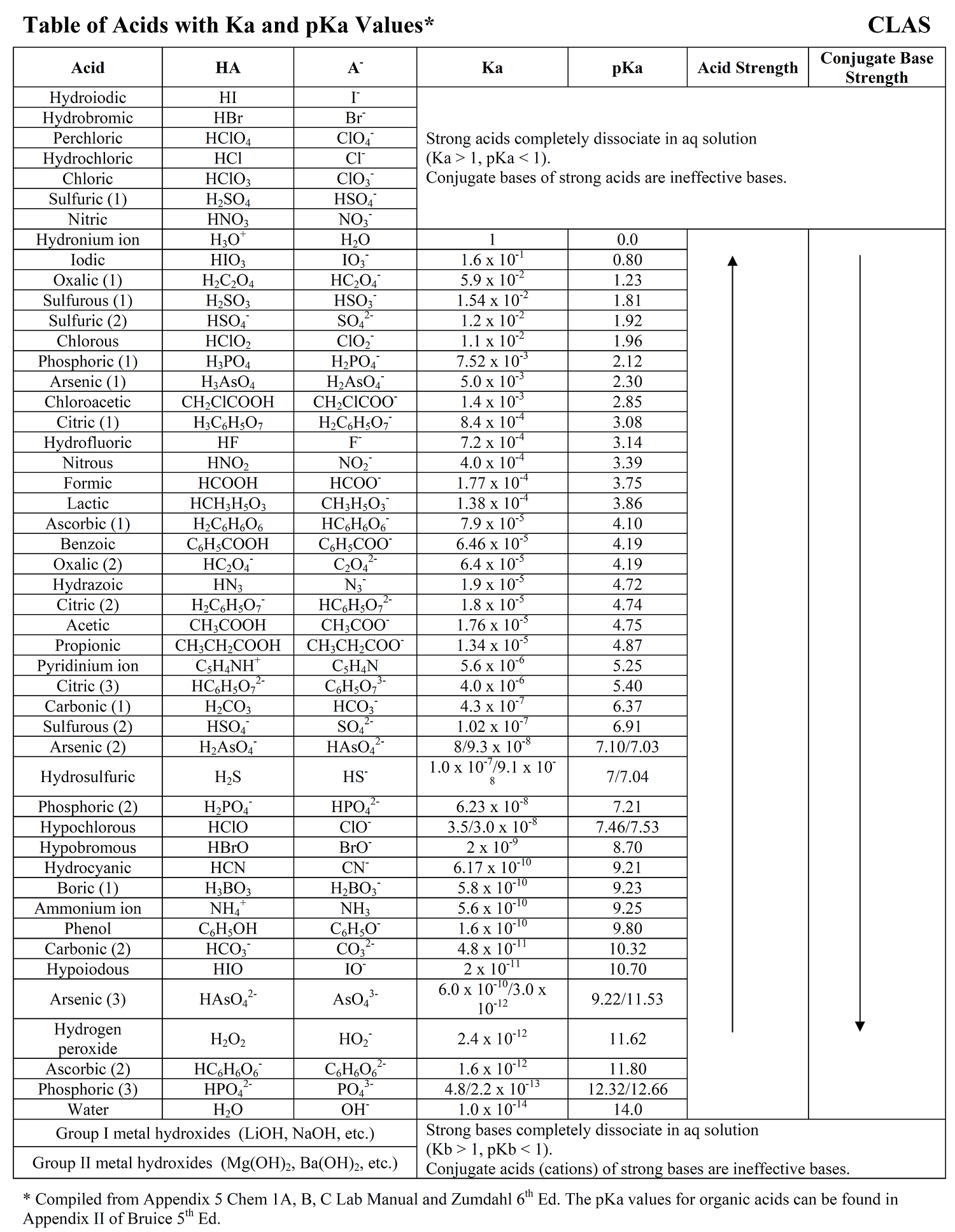
Ka, Kb. Comparing the pH of two acids 1.Predict the pH of HCl and HF (below) 2.Calibrate a pH meter 3.Measure the pH of HCl(aq) and HF(aq) 4.Complete. - ppt download

Reaction Kinetics of HCl Catalytic Oxidation over a Supported Cu-Based Composite Industrial Catalyst | Industrial & Engineering Chemistry Research
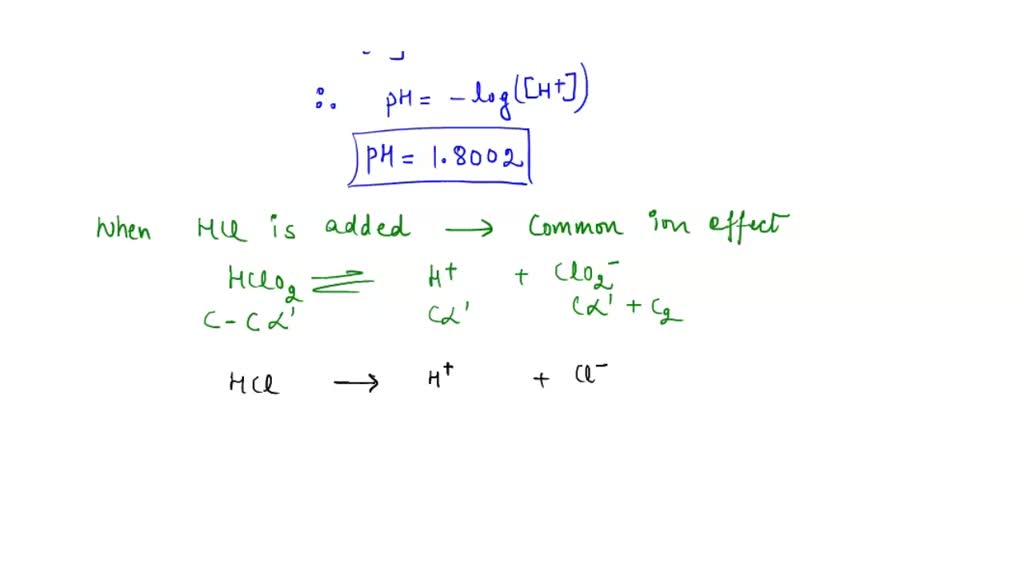
SOLVED: Determine the pH of a solution that is 0.00424 M HCl and 0.0228 M HClO2. The Ka of HClO2 is 1.1×10âˆ'2.

Binding Association Constants (Ka ) and Binding Sites (N) for Three... | Download Scientific Diagram